Images courtesy of Joe Paulisin, DO, Grand Blanc, MI

.png)

Safety
Independently Adjudicated Safety Results
Excellent long-term outcomes demonstrate the safety and effectiveness of wall-to-wall clot removal with the ClotTriever Thrombectomy System. 1,2,3
Procedural outcomes from the 500 patients enrolled in CLOUT registry demonstrate the proven safety profile of the ClotTriever system. 2

*Serious adverse events (SAEs) adjudicated by an independent medical monitor
Low rethrombosis/residual thrombus rate at 30 days3

Effectiveness
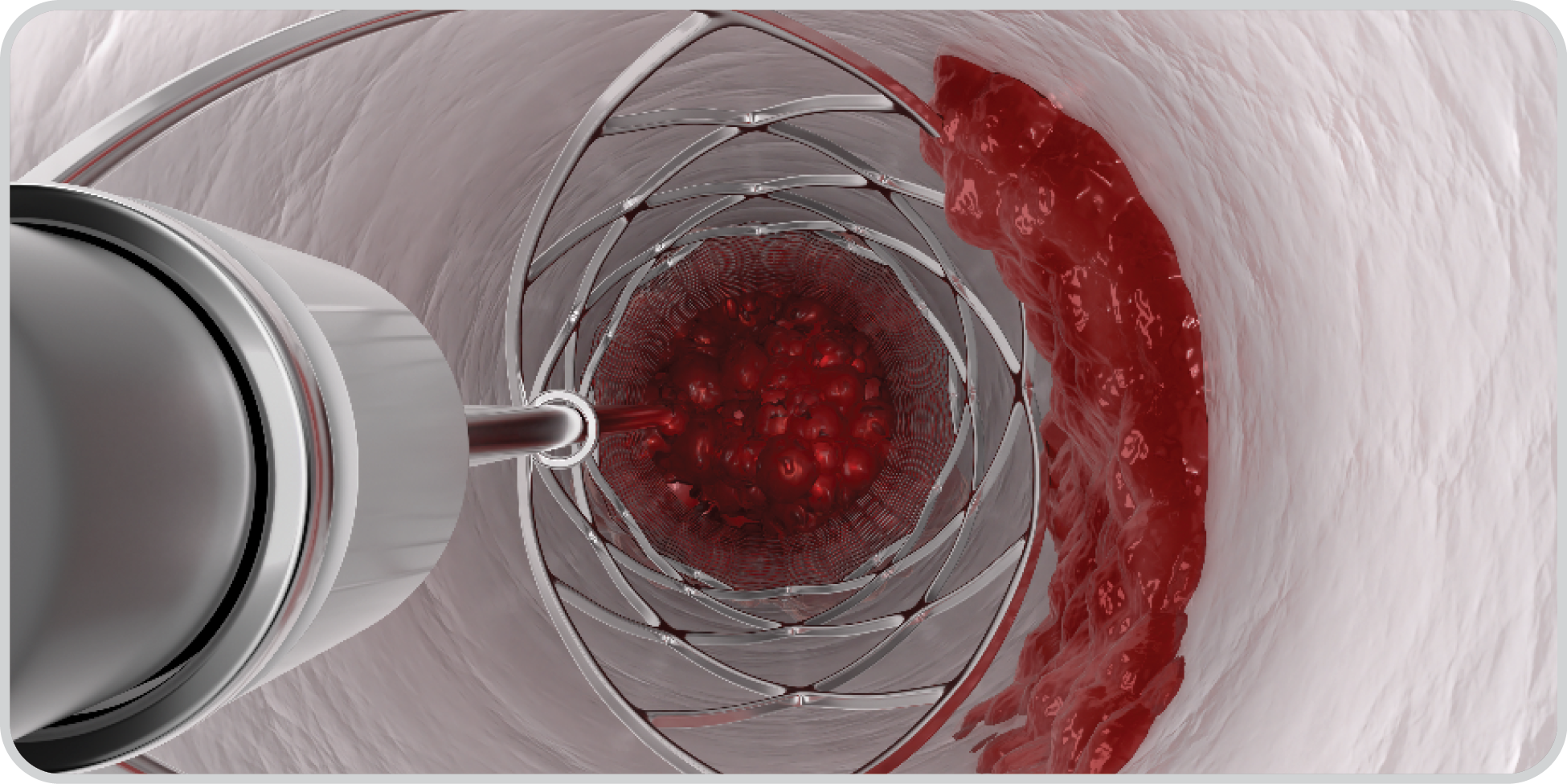
Wall-to-Wall Clot Removal
Only the ClotTriever catheter delivers wall-to-wall clot removal to clear thrombus obstruction and restore valvular function. 2
.png)
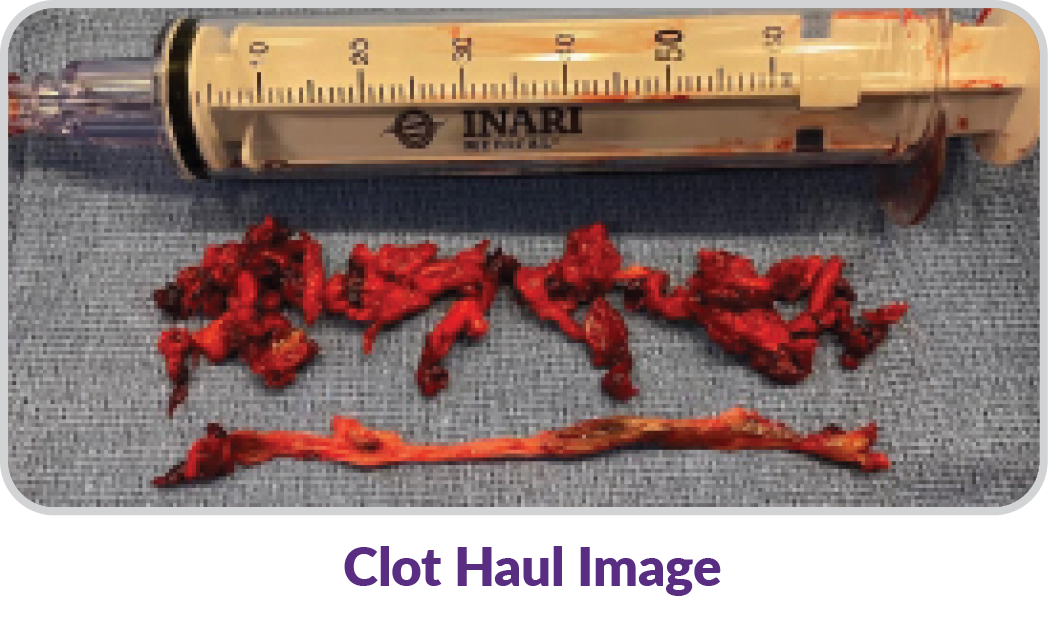
Wall-to-wall clot removal with ClotTriever removes the full range of clot chronicity.
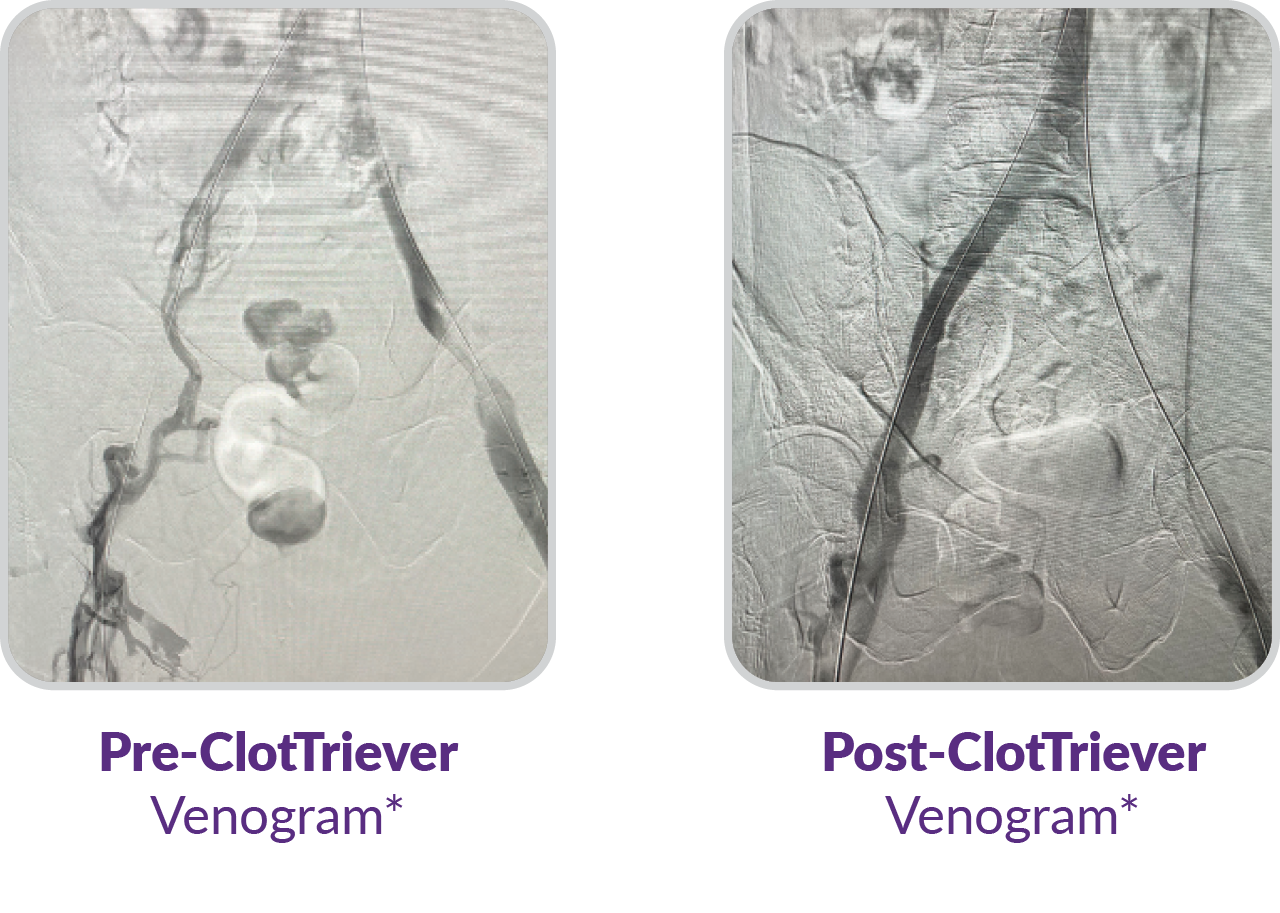
Aspiration alone is associated with residual thrombus.4
Chronic venous hypertension by residual venous obstruction and valvular reflux are assumed to play a major role in the development of post-thrombotic syndrome (PTS).5
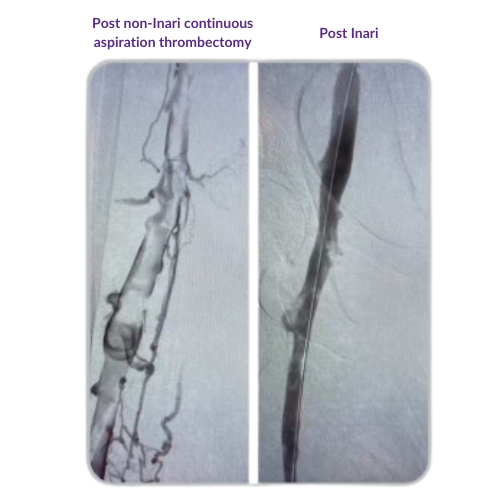
Non-Inari continuous aspiration thrombectomy residual thrombus case example: Post-procedure venogram captured 3 days after intervention (left). Post-procedure ClotTriever venogram shows clearance of residual thrombus and restoration of blood flow (right).*
Image courtesy of treating physician. Data on file
Evidence
ClotTriever is the only mechanical thrombectomy device with long-term outcomes
PTS symptom severity, by Villalta Score
(P < 0.0001)
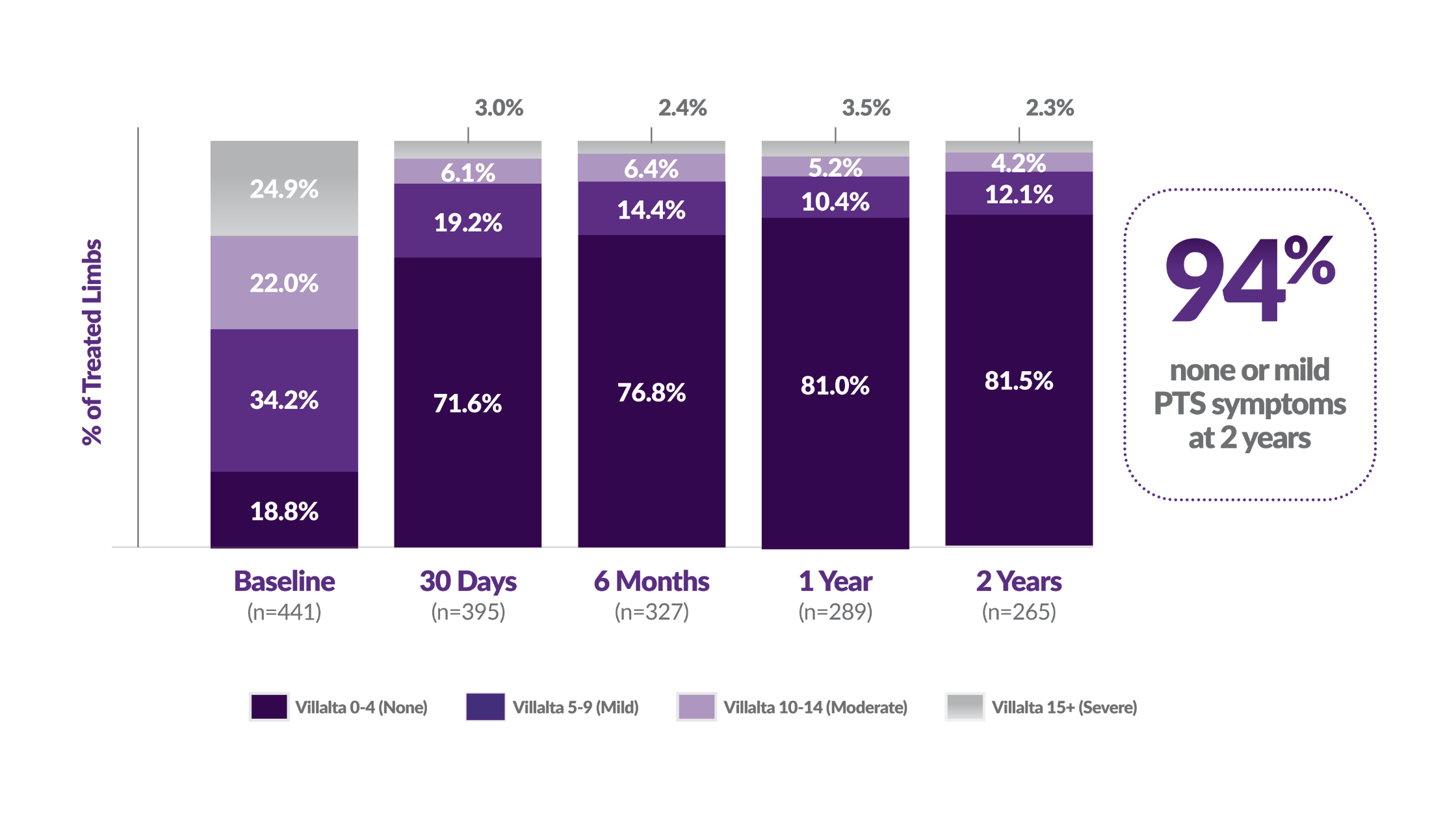
Intervention with ClotTriever leads to low PTS rates at 2-years 4, 5, 6
Moderate or severe PTS rates at 2-years in major DVT studies
Data provided for context only. Not meant to be directly comparable.
.png)
* ≥75% thrombus removal assessed via Marder score by an independent core laboratory
CAVA:
Study Design – 184 patients enrolled at 15 hospitals in the Netherlands, multicenter, randomized, single‐blind, allocation‐concealed, parallel‐group, superiority trial designed to assess the impact of additional ultrasound‐accelerated catheter‐directed thrombolysis compared with standard post‐thrombotic management on the development of PTS after acute iliofemoral DVT. Patients 18 to 85 years of age with first time iliofemoral DVT and maximum symptom duration of 14 days were randomized in a 1:1 ratio to standard anticoagulation therapy or standard anticoagulation therapy with additional ultrasound-accelerated catheter-directed thrombolytics. Regular follow-up visits were performed at clinic 3, 6, 12 months after inclusion and annually thereafter if preferred by the patient.
Primary outcome – The proportion of patients with PTS during follow‐up later than 12 months. The trial did not show a reduction of post‐thrombotic syndrome (PTS) after additional ultrasound‐accelerated catheter‐directed thrombolysis in patients with acute iliofemoral deep vein thrombosis at 1‐year follow‐up.
ATTRACT
Study Design – A phase 3, 692 patients enrolled at 56 clinical sites in the US, multicenter, randomized, open-label, assessor-blinded, controlled clinical trial sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) designed to assess the development of post-thrombotic syndrome between 6 and 24 months of follow-up. Patients 18 to 75 years of age with proximal DVT and maximum symptom duration of 14 days were randomized in a 1:1 ratio to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis (catheter-mediated or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, with or without stenting).
Primary outcome – The development of post-thrombotic syndrome defined at Villata score 5 or higher between 6 and 24 months of follow-up. The trial showed no significant between-group difference in the percentage of patients with the post-thrombotic syndrome.
References:
- Bisharat, et al. One-Year Clinical Outcomes Following Mechanical Thrombectomy for Deep Vein Thrombosis: A CLOUT Registry Analysis. JSCAI. 2024.
- Shaikh, et al. Six-Month Outcomes of Mechanical Thrombectomy for Treating Deep Vein Thrombosis: Analysis from the 500-Patient CLOUT Registry.
Cardiovasc Int Rad. 2023. - Dexter, D. Interim two year outcomes from the fully enrolled CLOUT registry. Presented at AVF 2024 (Tampa, FL).
- Vedantham S, et. al., ATTRACT Trial Investigators. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017
- Notten P, et. al., CAVA (Ultrasound-Accelerated Catheter-Directed Thrombolysis on Preventing Post-Thrombotic Syndrome) Trial: Long-Term Follow-Up Results. J Am Heart Assoc. 2021.
- Dexter, D. Final Two-Year CLOUT Outcomes. Presented at VIVA 2024 (Las Vegas, NV)
Indications for Use: The ClotTriever thrombectomy system is indicated for (1) the non-surgical removal of thrombi and emboli from blood vessels; and (2) injection, infusion and/or aspiration of contrast media and other fluids into or from a blood vessel. The ClotTriever thrombectomy system is intended for use in the peripheral vasculature, including deep vein thrombosis (DVT).
Refer to instructions for Use for complete indications for use, contraindications, warnings, and precautions.
For all non-Inari products, please refer to manufacturer Instructions for Use/Intended Purpose for complete indications for use, contraindications, warnings and precautions.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
All trademarks are property of their respective owners.
PRO-1783-USA-EN-v3

Inari Medical, Inc. Headquarters
6001 Oak Canyon, Suite 100
Irvine, CA 92618, United States
Connect
Follow Us